Monday, September 13, 2004
Sorting Antidepressant Evidence is Tough
The US Food and Drug Administration started their review
of the data regarding the risk of suicidal behavior in children and
adolescents who are treated with antidepressant medication. The
meeting started today and ends tomorrow (9/14/2004). I checked on
the FDA site to see if they have posted any updated information, but
apparently they don't have any bloggers on their staff, because there
is nothing new yet -- ever though they adjourned for the day
several hours ago.
Google lists 133 news articles already. The best title I saw was this one:
Other article titles are : FDA Panel Debates Suicide Risk for Kids On Antidepressants; Mom Credits Prozac with Saving Child's Life; Reviewer Says Depression Drugs, Suicide Linked. Notice that the titles span a spectrum from "Yes, there is a risk;" to "The risks are debatable;" to "We can't tell if there is a risk;" up to "These drugs saved my child." The consumer, meanwhile, has to sort this out.
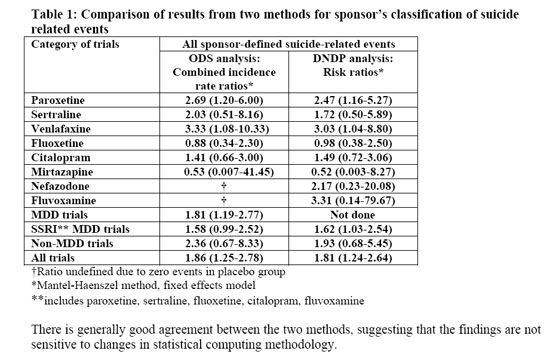
I did not read all 133 articles, but a brief survey indicates that there is no new information yet, compared to the material already available. The FDA posted the briefing materials for the meeting ahead of time. There are two pages: 1 2. Most of the information is on links from the first page. Much of the information is esoteric; for example, here is a table from a summary PDF document:
The two data columns refer to the original statistical analysis of the data done by the Office of Drug Safety. When there was a lot of political turmoil, the FDA asked a group at Columbia University to reclassify the data regarding "suicide-related" adverse events. This was done because it is not always a simple matter to classify such events. For example, if someone takes five aspirin tablets, is that a suicide attempt? The reason for analysis of suicide related events, rather than actual suicide, is that there were no actual suicides in any of the studies. The Division of Neuropharmacological Drug Products then analyzed the data again. The results are roughly comparable.
OK, the data are comparable. But what do they mean? A risk ratio is a number that indicates relative risk, not absolute risk. The number "2.69," for example, in the ODS analysis of the date for paroxetine (Paxil) shows that for every one suicide-related event per unit of time in the placebo group, there were 2.69 such events in the group that took the active drug. The numbers in parentheses denote the confidence interval. For examples of how these numbers can be misinterpreted, follow the link to the Wikipedia article on confidence intervals. Unfortunately, the text of the article does not say what level of confidence is indicated by the intervals, but they probably mean 90% confidence. For the paroxetine data in the ODS analysis, the numbers indicate that we can be 90% sure that the actual risk ratio is between 1.20 and 6.00. In other words, there is only a 5% chance that the relative risk is less than 1.2. Therefore, it is reasonable to conclude that this represents a real increase in risk.
Unfortunately, the real question here cannot be answered with the data. That is: is there a correlation between the suicide-related behaviors observed, and any risk of actual suicide? Until we know more, I think we have to assume that there is a correlation, but it would be wise to remember that this has not been proven. At first, it may seem like a no-brainer, in that it seems so plausible to assume that there would be a direct connection. Remember, though, that we are talking about life-or-death situations here, so we really would like to know for sure.
Since right now the evidence for the effectiveness of antidepressant medication, for the treatment of major depression in children, is weak, probably most consumers would want to avoid such use in the treatment of mild or moderate cases. In more severe cases, one would want to exercise caution, but, depending on a variety of factors, decide to go ahead with it. This probably is what the FDA will conclude, once they finally get around to making a clear statement of the subject.
Google lists 133 news articles already. The best title I saw was this one:
Sorting antidepressant evidence is tough
Dallas Morning News (subscription), TX -2 hours ago
By KAREN PATTERSON / The Dallas Morning News. Drug regulators are ready to make hard decisions about the safety of antidepressant medicines. ...
Dallas Morning News (subscription), TX -
By KAREN PATTERSON / The Dallas Morning News. Drug regulators are ready to make hard decisions about the safety of antidepressant medicines. ...
Other article titles are : FDA Panel Debates Suicide Risk for Kids On Antidepressants; Mom Credits Prozac with Saving Child's Life; Reviewer Says Depression Drugs, Suicide Linked. Notice that the titles span a spectrum from "Yes, there is a risk;" to "The risks are debatable;" to "We can't tell if there is a risk;" up to "These drugs saved my child." The consumer, meanwhile, has to sort this out.
I did not read all 133 articles, but a brief survey indicates that there is no new information yet, compared to the material already available. The FDA posted the briefing materials for the meeting ahead of time. There are two pages: 1 2. Most of the information is on links from the first page. Much of the information is esoteric; for example, here is a table from a summary PDF document:
The two data columns refer to the original statistical analysis of the data done by the Office of Drug Safety. When there was a lot of political turmoil, the FDA asked a group at Columbia University to reclassify the data regarding "suicide-related" adverse events. This was done because it is not always a simple matter to classify such events. For example, if someone takes five aspirin tablets, is that a suicide attempt? The reason for analysis of suicide related events, rather than actual suicide, is that there were no actual suicides in any of the studies. The Division of Neuropharmacological Drug Products then analyzed the data again. The results are roughly comparable.
OK, the data are comparable. But what do they mean? A risk ratio is a number that indicates relative risk, not absolute risk. The number "2.69," for example, in the ODS analysis of the date for paroxetine (Paxil) shows that for every one suicide-related event per unit of time in the placebo group, there were 2.69 such events in the group that took the active drug. The numbers in parentheses denote the confidence interval. For examples of how these numbers can be misinterpreted, follow the link to the Wikipedia article on confidence intervals. Unfortunately, the text of the article does not say what level of confidence is indicated by the intervals, but they probably mean 90% confidence. For the paroxetine data in the ODS analysis, the numbers indicate that we can be 90% sure that the actual risk ratio is between 1.20 and 6.00. In other words, there is only a 5% chance that the relative risk is less than 1.2. Therefore, it is reasonable to conclude that this represents a real increase in risk.
Unfortunately, the real question here cannot be answered with the data. That is: is there a correlation between the suicide-related behaviors observed, and any risk of actual suicide? Until we know more, I think we have to assume that there is a correlation, but it would be wise to remember that this has not been proven. At first, it may seem like a no-brainer, in that it seems so plausible to assume that there would be a direct connection. Remember, though, that we are talking about life-or-death situations here, so we really would like to know for sure.
Since right now the evidence for the effectiveness of antidepressant medication, for the treatment of major depression in children, is weak, probably most consumers would want to avoid such use in the treatment of mild or moderate cases. In more severe cases, one would want to exercise caution, but, depending on a variety of factors, decide to go ahead with it. This probably is what the FDA will conclude, once they finally get around to making a clear statement of the subject.
(Note: The Rest of the Story/Corpus Callosum has moved. Visit the new site here.)
E-mail a link that points to this post:
Comments:
Post a Comment