Wednesday, January 19, 2005
XP13512: Drug Development News
Most often, when you see articles about drugs with names such as XP13512, chances are that the drug never will sit on a pharmacy shelf.
In an earlier life, I was told that in some relatively nonindustrialized cultures, infants were not given names at birth. Infant mortality was so high, that the parents would wait until the child attained an age that gave a good chance of survival. Only then was it considered safe to give the child a name.
People in the drug development business will say that bringing a drug to market is a lot like giving birth. Except it takes a lot longer. Like the nonindustrialized peoples, the drug companies think it is bad luck to give real names to compounds that might not make it. So, they come up with names that imply no emotional attachment, names like XP13512.
XP13512 has a pretty good chance of making it, despite having no real name. It is a prodrug of gabapentin. A little background: Gabapentin (Neurontin) was a blockbuster drug for Pfizer, but went generic late last year. That means that sales are falling quickly. Pfizer happened to have a new drug, pregabalin, in the pipeline. It should be on pharmacy shelves later this year, with the brand name Lyrica. I haven't reviewed the studies on pregabalin, although I probably will soon, and post a bit about it. From Pfizer's website:
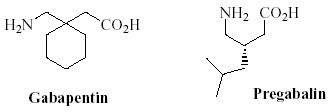
Today, I am more in the mood to write about pharmacology, otherwise known as "the fun stuff." Pregabalin was made by modifying the chemical structure of gabapentin. As the blurb above notes, this resulted in greater potency, and they claim a lower incidence of adverse effects.
Another company appears to have found a different approach. Xenoport -- a relatively small player in the industry -- developed a prodrug, XP13512. A prodrug is a drug that may not be active in its native form, but which is changed chemically to an active compound, when it is absorbed by the patient.
What Xenoport did, was to develop a compound that is absorbed more readily than gabapentin, which then is converted to an active compound. This will not solve the problem of patients who develop adverse effects, but it does mean that it will be feasible to attain much higher blood levels, if necessary. Those patients who are willing and able to tolerate higher concentrations may be able to get more benefit. Also, since the absorption is so much better, the pills won't have to be so large.
Pregabalin will get to the market first, which is an advantage with respect to marketing. Although it is similar to gabapentin, it will be possible to market it as "the new and improved Neurontin." It seems that that marketing strategy only works once, though, XP13512 is likely to be seen as a "me too" drug, an appellation that has no market appeal.
Is there anything that Xenoport can do to overcome this? Back to pharmacology: The main indication for use of XP13512, like that of pregabalin, will be the treatment of pain. However, drugs often have more than one purpose. Xenoport knows that the current treatment of choice for restless legs syndrome, pramipexole (Mirapex), has the unappealing adverse effect of causing the unpredictable sudden onset of sleep in a small percentage of patients. If they can demonstrate that XP13512 is effective for RLS, but without that particular adverse effect, they can, at least partly, shake off that "me too" label. They have reported success in a phase II trial for treatment of RLS.
Assuming that XP13512 is approved by the FDA, this will create an interesting situation: there will be three similar drugs on the market: gabapentin, which will be inexpensive, but will have the limitations noted above; pregabalin, which may be just as effective, but more expensive, and may have a lower incidence of adverse effects; and XP13512, which will have the same adverse effect profile as gabapentin, and will be expensive, but which can be given in smaller pills, with the added advantage that higher blood levels can be achieved.
For those of us who like to watch races, but think NASCAR wastes too much gasoline, it will be fun to see how the competition between gabapentin, pregabalin, and XP13512 plays out.
In an earlier life, I was told that in some relatively nonindustrialized cultures, infants were not given names at birth. Infant mortality was so high, that the parents would wait until the child attained an age that gave a good chance of survival. Only then was it considered safe to give the child a name.
People in the drug development business will say that bringing a drug to market is a lot like giving birth. Except it takes a lot longer. Like the nonindustrialized peoples, the drug companies think it is bad luck to give real names to compounds that might not make it. So, they come up with names that imply no emotional attachment, names like XP13512.
XP13512 has a pretty good chance of making it, despite having no real name. It is a prodrug of gabapentin. A little background: Gabapentin (Neurontin) was a blockbuster drug for Pfizer, but went generic late last year. That means that sales are falling quickly. Pfizer happened to have a new drug, pregabalin, in the pipeline. It should be on pharmacy shelves later this year, with the brand name Lyrica. I haven't reviewed the studies on pregabalin, although I probably will soon, and post a bit about it. From Pfizer's website:
Once generic manufacture of gabapentin is approved, sales of Pfizer's Neurontin are likely to fall dramatically. Encouraging physicians to switch patients from Neurontin to pregabalin, if approved, will be an important strategy in minimising the impact of generic competition. Pregabalin is as effective as Neurontin, but at lower doses, which translates to fewer side effects. Thus, it is well placed to capture Neurontin's market share.One of the factors that limits the usefulness of Neurontin is its low potency. Patients often have to take as much as 3.6 grams (six large tablets) to get an effect. But there is a second problem. The human intestine typically cannot not absorb more that 3.6 grams per day. Thus, for most patients, there is no point in trying higher doses. There is more interesting stuff to say about Neurontin and Lyrica, but that is not the point today.
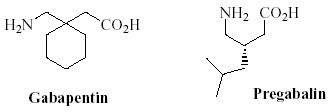
Today, I am more in the mood to write about pharmacology, otherwise known as "the fun stuff." Pregabalin was made by modifying the chemical structure of gabapentin. As the blurb above notes, this resulted in greater potency, and they claim a lower incidence of adverse effects.
Another company appears to have found a different approach. Xenoport -- a relatively small player in the industry -- developed a prodrug, XP13512. A prodrug is a drug that may not be active in its native form, but which is changed chemically to an active compound, when it is absorbed by the patient.
What Xenoport did, was to develop a compound that is absorbed more readily than gabapentin, which then is converted to an active compound. This will not solve the problem of patients who develop adverse effects, but it does mean that it will be feasible to attain much higher blood levels, if necessary. Those patients who are willing and able to tolerate higher concentrations may be able to get more benefit. Also, since the absorption is so much better, the pills won't have to be so large.
Pregabalin will get to the market first, which is an advantage with respect to marketing. Although it is similar to gabapentin, it will be possible to market it as "the new and improved Neurontin." It seems that that marketing strategy only works once, though, XP13512 is likely to be seen as a "me too" drug, an appellation that has no market appeal.
Is there anything that Xenoport can do to overcome this? Back to pharmacology: The main indication for use of XP13512, like that of pregabalin, will be the treatment of pain. However, drugs often have more than one purpose. Xenoport knows that the current treatment of choice for restless legs syndrome, pramipexole (Mirapex), has the unappealing adverse effect of causing the unpredictable sudden onset of sleep in a small percentage of patients. If they can demonstrate that XP13512 is effective for RLS, but without that particular adverse effect, they can, at least partly, shake off that "me too" label. They have reported success in a phase II trial for treatment of RLS.
Assuming that XP13512 is approved by the FDA, this will create an interesting situation: there will be three similar drugs on the market: gabapentin, which will be inexpensive, but will have the limitations noted above; pregabalin, which may be just as effective, but more expensive, and may have a lower incidence of adverse effects; and XP13512, which will have the same adverse effect profile as gabapentin, and will be expensive, but which can be given in smaller pills, with the added advantage that higher blood levels can be achieved.
For those of us who like to watch races, but think NASCAR wastes too much gasoline, it will be fun to see how the competition between gabapentin, pregabalin, and XP13512 plays out.
(Note: The Rest of the Story/Corpus Callosum has moved. Visit the new site here.)
E-mail a link that points to this post:
Comments:
Post a Comment



